By Marnie Sanderson, Product Specialist – Sentia
Released in 2023 as the sixth test on the platform, Sentia’s acidity method measures the titratable acidity of wine and grape juice all in less than 60 seconds. The UBI patented test strip utilises a salt mediated reduction of H+ to H2, which generates an electrochemical signal that is captured by the Sentia device. Using machine-based learning models, the Sentia intelligence system then correlates this electrochemical signal with the industry recognised titration method commonly called titratable acidity.
However, within the wine industry the terminology used to describe this method is swapped interchangeably between total acidity and titratable acidity, regardless of the titration methods inability to capture the total amount of wine acids within the sample. Adding to the confusion is that the method used to calculate titratable acidity can also change depending on which part of the world you are from.
So how should the wine industry define and describe our acidity measurements in wine, and what are the differences between titratable acidity measurements around the globe?
Definition and measurable differences of total acidity and titratable acidity
There are some slight but significant distinctions between the terms titratable acidity and total acidity that are important to define, in order to understand why confusion arises around these terms when used synonymously.
The definition differences
Titratable acidity measures the amount of carboxylic acid protons in a sample that can be recovered during titration with a strong base (Boulton, R. 1980). In the wine industry, the titrations end point differs usually as a function of the analyst’s regionality of work or education.
Total acidity quantifies all the acidic protons within a sample, both donated and undonated, and therefore captures the entirety of acids present. This can be performed using spectrometry or chromatography laboratory methods.
The definition differences between the two terms are a reason why using the term “total acidity” in place of “titratable acidity” for the general titration method, is technically incorrect.
The measurable differences
Regardless of the definition technicalities, a study by Boulton (1980) also found that the amount of acids recovered by titratable acidity at pH 7.0 was only 74% of the total acids present. To achieve full total acidity recovery, the sample must first be passed through a column with ion-exchange resin and all the carboxylic groups released before the titration step (Rajković, M. et al. 2007). This highlights further that an inherent difference exists between total and titratable acidity and is the second reason why using these two terms interchangeably can be inaccurate.
Measuring titratable acidity in the winery using traditional methods
As the acidic characteristics of juice and wine are determined by relatively weak organic acids (Torress, A.R. et al. 2011), the titration of wines with a strong base will provide a meaningful acidity measurement fit for winemaking purposes. Titratable acidity is also simple enough to be taught to inexperienced personnel and cheap to measure, which is another reason why it is the industry standard adopted for measuring acidity in wine and grape juice. There are also automated titration instruments such as the Hanna Automatic Mini Titrator 230V or the Metrohm 916 Ti-Touch that offer further simplicity if the budget allows.
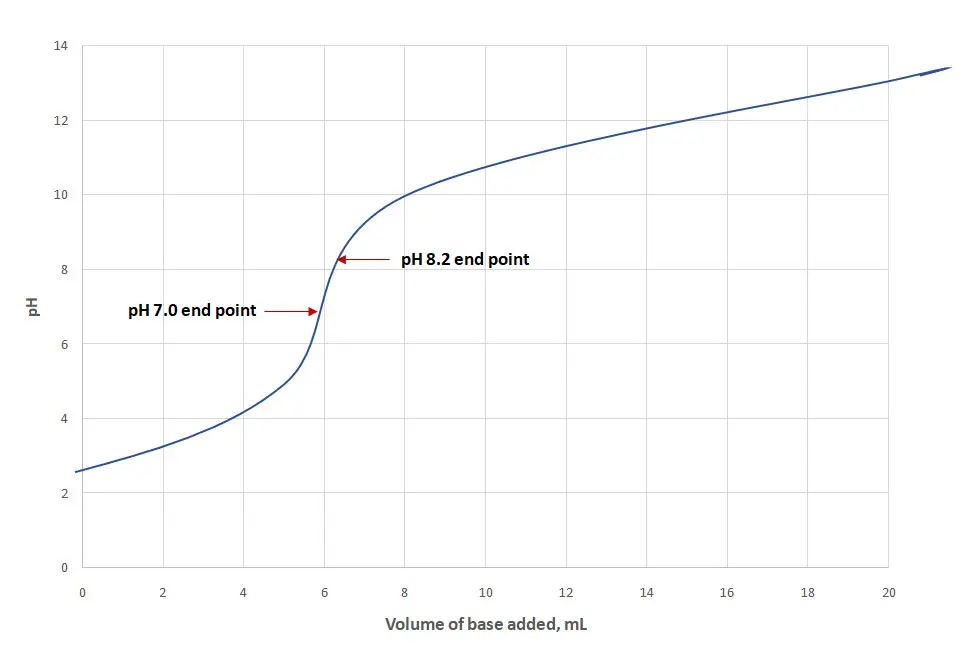
Regardless of whether you are using a manual or automatic titratable acidity method, all follow the same traditional principle. That is, slowly adding a strong base (usually sodium hydroxide) to a known amount of wine until it reaches a pre-determined end point which allows you to calculate the concentration of acids in the wine. In European countries and as shown in figure two, winemakers titrate to pH 7.0 and express the result as sulfuric acid. In other continents, winemakers titrate to pH 8.2 and express the result as tartaric acid. Results calculated to two different end points and expressed as different acids depending on your global location or educational and work influences, generates another layer of confusion.
Why do we measure at either titratable acidity at pH 7.0 expressed as sulfuric acid, or titratable acidity at pH 8.20 expressed as tartaric acid?
When titrating wine or juice with sodium hydroxide (the equivalent to titrating a weak acid with a strong base), the equivalence point occurs at a pH above 7.0 (Iland, P. et al. 2004). As pH 7.0 is where the dye indicator bromothymol blue changes colour, this is likely one reason historically that European nations measure to this end point.
Since wine acids are weak organic acids which are reluctant to donate their protons to a base at pH 7.0, some would argue that titrating to a higher pH of 8.2 is more correct, as this is where come donation is complete (FIVS, 2015). For this reason, and the fact a phenolphthalein indicator turns pink at this range as well, many of us choose a pH of 8.2 as our chosen end point when measuring titratable acidity.
As different amounts of base are used to reach each endpoint, the difference in acidity of the same wine being titrated to pH 7.0 and 8.2 is around 6-8% (Boulton, R. 1980). Therefore, comparing a USA based Chardonnay with USA titratable acidity measurement of 6.0 g/L has a very different meaning (and acidity level) to a Riesling grown in Germany with a European titratable acidity measurement of 6.0 g/L as well.
Adding further complexity is that titratable acidity is expressed as though all the available protons were donated from one acid species. Those who measure titratable acidity to pH 7.0 usually express it in units of sulfuric acid, whilst those that measure to pH 8.20 express it in units of tartaric acid, which is the acid of highest concentration in grapes. Nevertheless, both expressions are incorrect and misleading, as the acids encompassed by the titration method incorporates many organic acids.
The major acids captured during wine and grape juice acidity measurements
There are many acids found in wine and grape juice besides tartaric acid, some more prevalent than others. When using Sentia or any titration-based method (even if you call it total acidity) you can expect the following acids to be captured:
- Tartaric acid
- Malic acid
- Succinic acid
- Lactic acid
- Acetic acid
- Citric acid
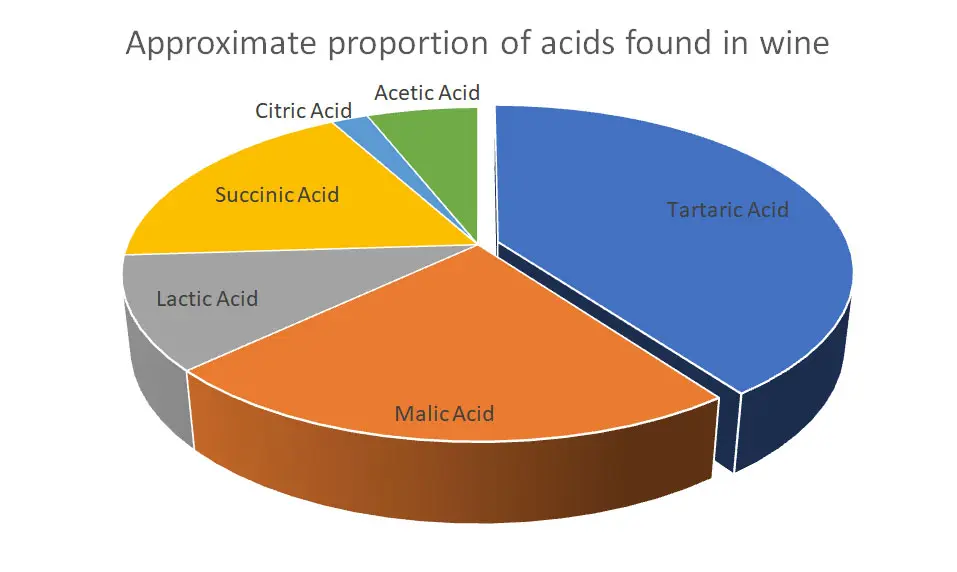
Of these acids captured, tartaric and malic acids account for the majority within a wine (see figure three). Some studies such as that by Volmer found they accounted for over 90% (2007), whilst another at the Australian Wine Research Institute found this number is more like 60% (Wilkes, E. 2016).
Using the two terms total acidity and titratable acidity interchangeably
Regardless of the technical definition differences, the two terms, titratable and total are often used synonymously, even when referring to the same industry standard titration method. That is, titrating a volume of wine with a basic solution to get to a particular pH end point.
For example, the recommended method provided by the International Organization of Vine and Wine (a European based group), calls the measurement of acids in wine total acidity. However, by its method description, it recommends a titration to a pH 7.0 end point. As this method would not recover all the hydrogen ions expected from the acids of grape juice and wine (Boulton, R. 1980), it is understandable then, why calling the titratable acidity method “total acidity” causes confusion for those of us who like chemical definitions.
However, at the end of the day, there is a relatively simple rule to go by.
If you are comparing Sentia to your existing method of measuring acidity, you should consider the chemical reaction occurring. It is likely, regardless of whether you define your historical reference measurement as titratable acidity or total acidity, that you are still titrating your sample with a strong base (or an equivalent method). The Sentia acidity measurement correlates to this titration method in all corners of the globe – that is and as shown in figure one, to the historically used end points of pH 7.0 and 8.2 by a simple selection in the test type screen.
References
Boulton, R. (1980). The Relationship Between Total Acidity, Titratable Acidity and pH in Wine. American Journey of Enology and Viticulture, 31(1), 76 – 80.
Iland, P., Bruer, N., Edwards, G., Weeks., S & Wilkes, E. (2004). Chemical Analysis of Grapes and Wine: Techniques and Concepts. Patrick Iland Wine Promotions Pty Ltd.
FIVS. (2015). Harmonizing Expression of Measurement Results in Wine Analysis: Testing for Total or Titratable Acidity (TA) of Wine. International Wine Technical Summit, Technical Brief.
International Organisation of Vine and Wine. (2023). Compendium of International Methods of Wine and Must Analysis, Volume One. International Organisation of Vine and Wine.
Rajković, M., Novaković, I., & Petrović, A. (2007). Determination of Titratable Acidity in White Wine. Journal of Agricultural Sciences, 52(2), 169 – 184.
Torres, A.R., Lyra, W., Andrade, S., Andrade, R., Silva, E., Araújo, M., & Gaiao, E. (2011). A Digital Image-based Method for Determining of Total Acidity in Red Wines Using Acid-base Titration Without Indicator. Talanta, 84, 601 – 606.
Volmer, D., Curbani, L., Parker, T., Garcia, J., Schultz L., & Borges, E. (2017). Determination of Titratable Acidity in Wine Using Potentiometric, Conductive, and Photometric Methods. Journal of Chemical Education, 94, 1296 – 1302.
Wilkes, E. (2016). Wine Acids, Not Just Tartaric. Technical Review No. 221. The Australian Wine Research Institute, 10 – 13.